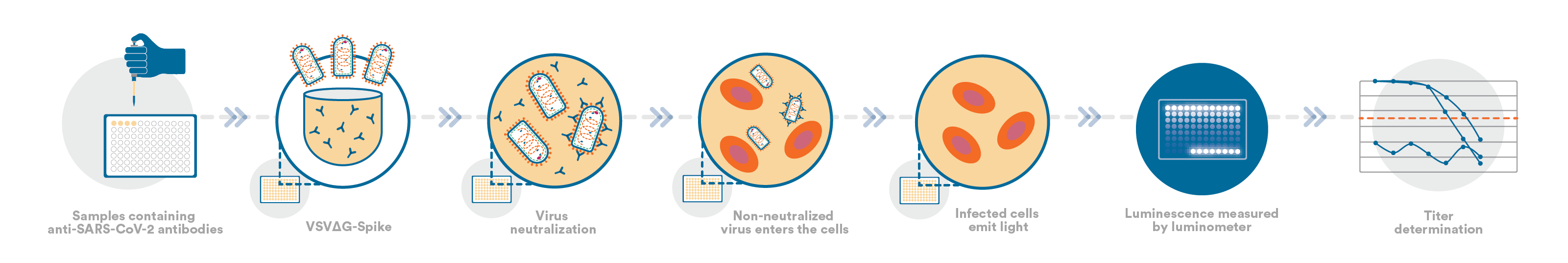

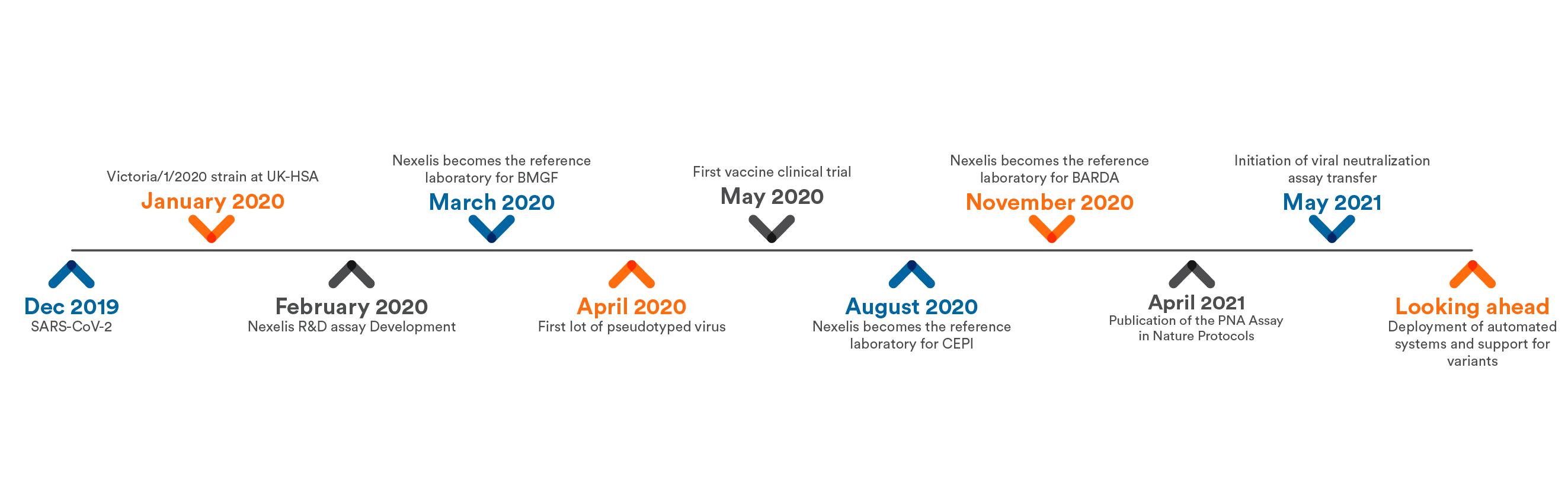
Leading The Way: Using Advanced Assays to Fight SARS-CoV-2
The outbreak of the SARS-CoV-2 virus caused a sudden shift in focus for the biopharmaceutical industry. After the genetic sequence of the virus was released in early 2020, vaccine manufacturers...
Where the Science Leads Us: SARS-CoV-2
By March 2020, when the World Health Organization declared SARS-CoV-2 a pandemic, every major multinational pharmaceutical company, innovative biotechnology company, governmental, and nongovernmental...

Interlaboratory Comparative Study of HAI Assays
Influenza vaccine development is a unique paradox. A seasonal influenza formulation is constructed from four strains selected by organizations such as WHO and CDC. The strain selection is then...

Taking a biomarker-led approach to R&D
Leveraging biomarkers in the clinical field isn’t a new concept. In fact, their value has been well documented in treatments for patients and in the development of new precision medicines. However,...

Industry collaboration is at an all-time high – but how do we take this beyond the COVID-19 pandemic?
The COVID-19 pandemic has seen exceptional collaboration amongst key industry partners including pharmaceutical companies, manufacturers, government and academia, and one major result of this...

Immuno-Oncology: Functional Assays for Immune Checkpoint Inhibitors as Emerging Therapeutics
In this recent webinar, immuno-oncology experts Sofie Pattijn, chief technology officer and co-founder of ImmunXperts, a Nexelis company, and Thibaut Janss, lead scientist, ImmunXperts, answer the...
Where the Science Leads Us: Bispecific Antibodies
There’s a reason that Nexelis is the leading specialty lab for what’s next in therapeutic testing and assay development. Our corporate DNA is built from scientific rigor, premium facilities, and the...
Living History: Our Role in Global Scientific Collaboration for SARS-CoV-2
In this recent webinar, Bassam Hallis, Ph.D., Head of Preclinical Development for Public Health England, and Luc Gagnon, VP of Vaccine Sciences for Nexelis, discuss pandemic preparedness and response...
